The
bovine viral diarrhea (BVD)
eradication in Denmark 1994-2002:
pilot studies, control and legislative measures, results, and extent and causes of new infections
eradication in Denmark 1994-2002:
pilot studies, control and legislative measures, results, and extent and causes of new infections
Viggo
Bitsch; DVM,
DVSc
viggo.bitsch@gmail.com
Abstract
Viggo
Bitsch: The bovine viral diarrhea (BVD) eradication in Denmark
1994-2002: pilot
studies,
control and legislative measures, results, and extent and causes of new infections.
control and legislative measures, results, and extent and causes of new infections.
In 1991, it was evident that eradicating Bovine Viral Diarrhea (BVD) was desirable. New Danish sensitive BVD antigen and antibody ELISAs and a subunit ISCOM BVD vaccine had been made at the State Veterinary Virus Research Institute. Still, their value had not been demonstrated, and methods to control and eradicate nationwide were never tested. The prevalence of the infection in cattle in Denmark was known to be very high. It was therefore decided to perform comprehensive pilot studies to document the efficacy of the new tests and to clarify how they could be used in the best possible way to test individual animals and determine the infection status of dairy and non-dairy herds. Additionally, a vaccination study would be conducted. Already in 1992, eradication was initiated in all herds in a geographically defined area - the Samsø island project - based on serological and virological tests of all animals in the herds. The main results from these pilot studies were that the vaccine was ineffective, but that the ELISAs were safe and well suited for an eradication program. A bulk tank milk reaction in the antibody blocking ELISA below 50 % of blocking would indicate BVD-free status, and an examination of just three young animals free from colostral antibodies, e,g., 8-10 months of age, could with acceptable reliability be used to indicate infection status and to confirm an earlier probably BVD-free status, and the island eradication study demonstrated that eradication was feasible.
The decision to start eradication nationwide was taken by the farmers’ organizations with effect from January 1994. It was a huge advantage that the Danish Dairy Board had its own laboratory experienced in large-scale control examinations and with any professional expertise. All test data were transferred to the central herd register, which was also administered by the farmers’ organizations.
After two years, the Danish Veterinary Service agreed to support the program with legal provisions, but eradication was not made obligatory. The most important legal regulations were that animals to be moved to other herds, exhibitions, or common pastures had to be accompanied by a certificate issued after blood testing of the individual animals and that persistently infected animals were not allowed in pastures. In 1999, the trade certificate conditions were relaxed for herds having been free for over two years so a blood test of animals to be moved was no longer required. From the course of the program, it was later concluded that it would have been far more effective and less expensive if legal provisions including an obligation to eradicate had been implemented right from the beginning. Nevertheless, a cost-benefit estimation later showed that expenses had been paid back within eight years.
Veterinary practitioners were placed centrally. At regular intervals, they received circulars and overviews of the situation in dairy and non-dairy herds in their practice including deadlines for confirmatory free-status control.
Persistent infection in earlier free herds was a severe problem over the whole period. Of 67 dairy herds found infected in 1997-1998 and investigated thoroughly, a few had become infected in a common pasture, for 39% of the herds persistently infected animals had been present at neighboring farms, but for 25% of the herds no immediate possible cause could be found. It was concluded that airborne transmission had to be responsible, not only for a high proportion of the infected herds where persistently infected animals were present on neighboring farms but also for a considerable part of the cases, where no immediate cause could be observed.
The most important experiences from the successful Danish BVD program are that the tests employed were supreme, that the lines selected for eradication and control were excellent, but also that eradication should have been compulsory right from the start. This condition would most likely have reduced the eradication phase of the program to four years.
1. Introduction
Denmark has a long history of control of infectious diseases in swine and cattle. Bovine brucellosis and bovine tuberculosis were officially eradicated 1959, and after that, a series of earlier widespread viral diseases, i.e., enzootic bovine leukosis (EBL, initiated 1959), Aujeszky's disease (AD, initiated gradually 1980-1982), infectious bovine rhinotracheitis (IBR, initiated 1984), and bovine viral diarrhea (BVD, initiated 1994), has also been eradicated [1]. Furthermore, the control of two also rather widespread bacterial diseases in cattle, bovine paratuberculosis (Verdugo et al., 2015) [2] and the Salmonella Dublin infection (Nielsen, 2013) [3] has so far been reasonably successful.
Regarding AD, the decision to eradicate was taken by the pig farmers’ associations, because of a severe change in the disease situation. Investigations had documented that new strains of Suid herpesvirus 1 of considerably higher virulence had developed from the indigenous virus strains. The new virus isolates, which could readily be differentiated from strains of lower virulence at the laboratory immediately on the diagnosis of an outbreak, furthermore showed a markedly higher tendency for airborne transmission to other herds, thus threatening the AD-free status of the elite breeding herds. The quick decision to initiate eradication, however, was not sufficiently coordinated with the state laboratories, so even if tests of very high and sufficient sensitivity had been developed and taken into use, the laboratories were not prepared for a sudden need for massive analyses. The program was significantly delayed, but the problems were overcome, and lessons were learned. The last outbreaks caused by native Danish virus strains were diagnosed in 1985, cf. review by Bitsch (2015) [4].
In
1984, when the milk quota system was introduced in the EU countries,
the cattle farmers' associations decided to eradicate IBR. A
practical point of view was that when the number of dairy cows had to
be reduced, preferably IBR-infected animals should be slaughtered.
Reliable tests suitable for both control of individual animals and
surveillance of infection status of non-dairy herds based on blood
testing, as well as for surveillance and control of the infection
status of dairy herds based on bulk tank milk testing were in place.
And of greatest importance was that these tests were well suited for
automation. Experiences had been gained from the eradication of IBR,
and main lines for eradication in affected herds, which actually
remained unchanged throughout the whole programme period, could
readily be given. The testing capacity necessary was established at a
private laboratory, later known as the Danish Cattle Health
Laboratory, owned by the Danish Dairy Board. Automation to a wide
extent, including the use of laboratory robots and electronic reading
and registration of results, was established here. Test results were
transferred to the Central Cattle Herd Register, from where relevant
test reports, as well as animal trade and herd certificates, were
issued and forwarded to the herd owners and/or their veterinary
practitioners.
In 1991, when Denmark had been cleared of both IBR and EBL[1], it was evident that eradication of BVD was desirable. But there were many questions. Eradication of BVD had never been attempted or even considered before in a country like Denmark, where the infection was widespread to such an extent that hardly any dairy herd was without antibody carriers. It was of utmost importance that the decision to eradicate should only be taken based on safe knowledge of effective and reliable control and eradication measures that would ensure a favorable outcome. The Danish Virus Research Institute had presented two ELISAs, one for detection of the virus in the leucocyte fraction of stabilized blood samples, and one for measurement of antibodies (Rønsholt et al., 1996) [5], but had also produced a killed vaccine that in a limited experiment had appeared promising, cf. Kamstrup et al. (1992) [6]. To evaluate the usefulness of these tests and the efficacy of the vaccine, it was decided to perform pilot studies on a relatively large scale. The necessary financial support was achieved, directly or indirectly, through the Danish Dairy Board.
In 1991, when Denmark had been cleared of both IBR and EBL[1], it was evident that eradication of BVD was desirable. But there were many questions. Eradication of BVD had never been attempted or even considered before in a country like Denmark, where the infection was widespread to such an extent that hardly any dairy herd was without antibody carriers. It was of utmost importance that the decision to eradicate should only be taken based on safe knowledge of effective and reliable control and eradication measures that would ensure a favorable outcome. The Danish Virus Research Institute had presented two ELISAs, one for detection of the virus in the leucocyte fraction of stabilized blood samples, and one for measurement of antibodies (Rønsholt et al., 1996) [5], but had also produced a killed vaccine that in a limited experiment had appeared promising, cf. Kamstrup et al. (1992) [6]. To evaluate the usefulness of these tests and the efficacy of the vaccine, it was decided to perform pilot studies on a relatively large scale. The necessary financial support was achieved, directly or indirectly, through the Danish Dairy Board.
2. BVD pilot studies
The three BVD pilot projects planned were (1) a vaccination study to verify the efficacy of a Danish killed BVD vaccine, and (2) a test evaluation study aiming at the clarification of the optimal ways to use blood samples and bulk tank milk samples to determine the infection status of various herds in a control and eradication scheme, and (3) a control and eradication experiment in a geographically defined area to document that eradication was possible.
2.1.
The vaccination study
In all. 50 BVD-free non-pregnant heifers from various herds were assembled on an empty farm in Jutland. After three weeks they were controlled and found to be still uninfected. The major part of the group was thereafter vaccinated with a subunit ISCOM vaccine (Kamstrup et al., 1992) [6] and checked serologically by the lines given by the virus research institute. Thereafter, all the heifers were oestrus-synchronized and artificially inseminated. Approximately two months later, a calf persistently infected with the BVD virus was introduced and placed with the heifers.
In all. 50 BVD-free non-pregnant heifers from various herds were assembled on an empty farm in Jutland. After three weeks they were controlled and found to be still uninfected. The major part of the group was thereafter vaccinated with a subunit ISCOM vaccine (Kamstrup et al., 1992) [6] and checked serologically by the lines given by the virus research institute. Thereafter, all the heifers were oestrus-synchronized and artificially inseminated. Approximately two months later, a calf persistently infected with the BVD virus was introduced and placed with the heifers.
Main conclusion
Most of the calves delivered by the vaccinated heifers were found persistently infected with BVD virus. It was, consequently, decided not to consider further the use of vaccination as a tool in the future control of BVD.
2.2.
The test evaluation study
This study involved all 369 dairy herds on the islands of Bornholm in the Baltic Sea and Mors in the Limfjord in Jutland. High sensitivities of the antigen and antibody ELISAs [5] were obtained by long-time incubation, close to 24 hours, at 37 oC, with the test samples in accordance with the regular antigen-antibody interaction lines documented by Bitsch, cf. review [7]. It should be recalled that antibody reactions in cows are approximately 25 times higher in late immunization phase serum samples than in the corresponding milk samples. Although the sensitivity of the antibody blocking ELISA would be somewhat lower than that of an indirect antibody ELISA also carried out with long-time incubation at 37 oC, the blocking modification was chosen, just as it was in the IBR eradication programme, for several reasons, i.e., (1) the sensitivity of the blocking modification is still very high, (2) it is more simple and therefore less expensive, as no control wells for individual test samples on the ELISA plates are included, (3) in spite of high sensitivity, false-positive reactions are practically non-existing, and finally, (4) plasma or serum samples with from mean to strong positive antibody reactions all give a nearly full blocking reaction, while for samples with low antibody concentrations the transition from almost no reaction (below 15% blocking) to almost full reaction (over 85% blocking) occurs gradually over an antibody concentration range corresponding to approx. 4 two-fold dilution steps, or a factor of 16. This modification would, therefore, be excellent for the quantification of the rather low antibody levels of special importance in bulk tank milk samples, without further titrations being needed.
Blood samples were collected from every herd according to the following lines: 2 from 3rd parity, 2 from 2nd parity, 2 from 1st parity cows, and 3 from calves aged 8-10 months. The cows were selected as described in order to enable an evaluation of the antibody blocking reaction in a bulk tank milk sample taken at the same time against the proportion of antibody-positive cows in the herd. To exclude reactions due to colostral antibodies, the age of the calves should be 8-10 months. The main results have been published, cf. Bitsch et al. (1994) [8], and Bitsch et al. (1997) [9].
All blood samples were tested for both virus and antibody. The herds were divided into four categories, I to IV, according to the results of the blood tests as follows, I: none or not more than 2 of the cows were antibody-positive; II: 3 or 4 cows were antibody-positive; III: 5 or all 6 cows but no calves were antibody-positive; and IV: the calves were antibody-positive, or if negative, virus-positive.
Figure 1 illustrates the correlation between the tank milk antibody reactions and both the herd category as defined above and the probability of persistent infection as estimated from the representative blood testings in the individual herds.
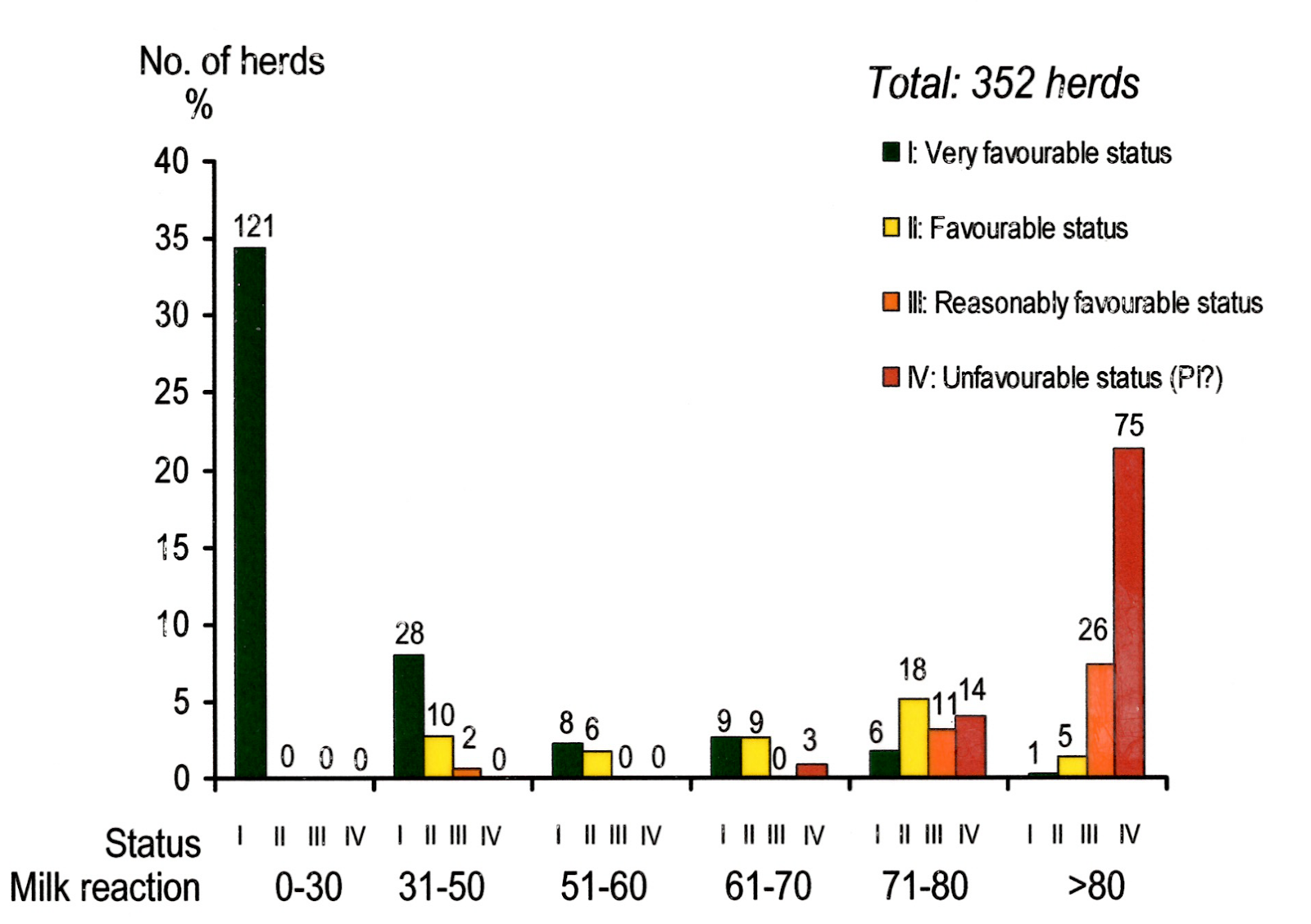
This study involved all 369 dairy herds on the islands of Bornholm in the Baltic Sea and Mors in the Limfjord in Jutland. High sensitivities of the antigen and antibody ELISAs [5] were obtained by long-time incubation, close to 24 hours, at 37 oC, with the test samples in accordance with the regular antigen-antibody interaction lines documented by Bitsch, cf. review [7]. It should be recalled that antibody reactions in cows are approximately 25 times higher in late immunization phase serum samples than in the corresponding milk samples. Although the sensitivity of the antibody blocking ELISA would be somewhat lower than that of an indirect antibody ELISA also carried out with long-time incubation at 37 oC, the blocking modification was chosen, just as it was in the IBR eradication programme, for several reasons, i.e., (1) the sensitivity of the blocking modification is still very high, (2) it is more simple and therefore less expensive, as no control wells for individual test samples on the ELISA plates are included, (3) in spite of high sensitivity, false-positive reactions are practically non-existing, and finally, (4) plasma or serum samples with from mean to strong positive antibody reactions all give a nearly full blocking reaction, while for samples with low antibody concentrations the transition from almost no reaction (below 15% blocking) to almost full reaction (over 85% blocking) occurs gradually over an antibody concentration range corresponding to approx. 4 two-fold dilution steps, or a factor of 16. This modification would, therefore, be excellent for the quantification of the rather low antibody levels of special importance in bulk tank milk samples, without further titrations being needed.
Blood samples were collected from every herd according to the following lines: 2 from 3rd parity, 2 from 2nd parity, 2 from 1st parity cows, and 3 from calves aged 8-10 months. The cows were selected as described in order to enable an evaluation of the antibody blocking reaction in a bulk tank milk sample taken at the same time against the proportion of antibody-positive cows in the herd. To exclude reactions due to colostral antibodies, the age of the calves should be 8-10 months. The main results have been published, cf. Bitsch et al. (1994) [8], and Bitsch et al. (1997) [9].
All blood samples were tested for both virus and antibody. The herds were divided into four categories, I to IV, according to the results of the blood tests as follows, I: none or not more than 2 of the cows were antibody-positive; II: 3 or 4 cows were antibody-positive; III: 5 or all 6 cows but no calves were antibody-positive; and IV: the calves were antibody-positive, or if negative, virus-positive.
Figure 1 illustrates the correlation between the tank milk antibody reactions and both the herd category as defined above and the probability of persistent infection as estimated from the representative blood testings in the individual herds.
Figure 1. BVD antibody blocking ELISA reactions in bulk tank milk from all dairy herds in two geographical regions as compared with the proportion of antibody-positive cows and with the probability of persistent infection.
The herds were divided into four categories according to results of age-group-representative blood testing (2 cows from each of 3 age groups were tested, see text). In category-IV herds, 8-10 months old calves had experienced the infection. The reaction interval from 15 to 85 percent blocking corresponds to an antibody concentration range varying by a factor of approx. 16. At the time where this English version of the figure was made, the number of active dairy herds in the two regions had been reduced from 369 to 352.
Main
conclusions
The most important results were (1) that a tank milk reaction below 60% blocking was clearly associated with a beneficial infection status with no persistently infected animals being present, so that later low tank milk reactions (below 50% blocking was later selected for the programme) could be used to confirm a true BVD-free status, (2) that herds with higher milk reactions would need further testing of representative blood samples in order to clarify, if persistently infected animals were present or not, and (3) that blood testing of 3 calves old enough to be free of colostral antibodies was an excellent tool for determining the herd infection status as being most probably BVD-free or not.
The most important results were (1) that a tank milk reaction below 60% blocking was clearly associated with a beneficial infection status with no persistently infected animals being present, so that later low tank milk reactions (below 50% blocking was later selected for the programme) could be used to confirm a true BVD-free status, (2) that herds with higher milk reactions would need further testing of representative blood samples in order to clarify, if persistently infected animals were present or not, and (3) that blood testing of 3 calves old enough to be free of colostral antibodies was an excellent tool for determining the herd infection status as being most probably BVD-free or not.
2.3.
The control and eradication study (the Samsø island project)
On
the island of Samsø there were 36 dairy and 77 non-dairy herds with
on average 70 and 15 animals over 3 months of age, respectively. All
farmers agreed to participate, and the project was started in early
1992.
Initially, all 3698 animals over 3 months of age were blood tested for virus and antibody. Nine dairy (25%) and 3 non-dairy herds (4%) had persistently infected animals. None of the dairy herds, but 23 of the non-dairy herds (30%) were without antibody carriers. All persistently infected animals were removed and slaughtered. The examination of the 2,200 blood samples from cows, cf. Figure 2. demonstrated convincingly that the antibody test could efficiently separate infected, antibody-positive animals from uninfected ones.
Figure 2. BVD antibody reactions in blood samples of all cows on the island of Samsø.
In the pilot eradication study, 2200 cows were tested for BVD antibody by a blocking ELISA.
Initially, all 3698 animals over 3 months of age were blood tested for virus and antibody. Nine dairy (25%) and 3 non-dairy herds (4%) had persistently infected animals. None of the dairy herds, but 23 of the non-dairy herds (30%) were without antibody carriers. All persistently infected animals were removed and slaughtered. The examination of the 2,200 blood samples from cows, cf. Figure 2. demonstrated convincingly that the antibody test could efficiently separate infected, antibody-positive animals from uninfected ones.
Figure 2. BVD antibody reactions in blood samples of all cows on the island of Samsø.
In the pilot eradication study, 2200 cows were tested for BVD antibody by a blocking ELISA.
The
ordinate indicates the percentage of animals. The results
convincingly demonstrate that a 50 percent blocking limit effectively
separated the animals into two groups, an infected and an uninfected
one.
Control on blood samples was thereafter conducted in all herds. Up to the spring of 1993, 715 and 367 animals from dairy and non-dairy herds, respectively, were tested, and the majority of herds was confirmed to be BVD-free. New persistently infected animals were found in 3 dairy herds, namely (1) a herd, where 6 animals had been found persistently infected at the first examination, (2) a herd where all animals at the first examination were antibody-positive, but 3 persistently infected calves had not been tested, because they were under 3 months of age, and (3) a herd where only a few cows and 2 heifers had been found antibody-positive, but where a persistently infected calf was born from one of the two antibody-positive heifers. So, 11 of the 36 dairy herds (31%) were persistently infected. No new persistently infected calves were found in the non-dairy herds.
It is of historical interest that the Samsø island study initiated 1992 was actually a combined project, as the blood samples and additional feces samples from the herds also were examined as part of a bovine paratuberculosis and Salmonella Dublin project at the Danish Cattle Health Laboratory. So, this Samsø island study was, in fact, the first step taken towards eradicating those two bacterial infections as well.
Control on blood samples was thereafter conducted in all herds. Up to the spring of 1993, 715 and 367 animals from dairy and non-dairy herds, respectively, were tested, and the majority of herds was confirmed to be BVD-free. New persistently infected animals were found in 3 dairy herds, namely (1) a herd, where 6 animals had been found persistently infected at the first examination, (2) a herd where all animals at the first examination were antibody-positive, but 3 persistently infected calves had not been tested, because they were under 3 months of age, and (3) a herd where only a few cows and 2 heifers had been found antibody-positive, but where a persistently infected calf was born from one of the two antibody-positive heifers. So, 11 of the 36 dairy herds (31%) were persistently infected. No new persistently infected calves were found in the non-dairy herds.
It is of historical interest that the Samsø island study initiated 1992 was actually a combined project, as the blood samples and additional feces samples from the herds also were examined as part of a bovine paratuberculosis and Salmonella Dublin project at the Danish Cattle Health Laboratory. So, this Samsø island study was, in fact, the first step taken towards eradicating those two bacterial infections as well.
Main conclusions
The Samsø island BVD study was planned for a period of three years, but the results already after a little more than one year were very promising. The ELISAs used were found to be sensitive and reliable. It was realized that the risk of introduction of the infection with traded pregnant animals, of contact transmission from neighbouring pastures, and airborne transmission from other herds, had been minimized because all herds on the island were included in the control project. But it was also concluded that a longer period would be needed to evaluate, to which extent reactivation of the infection in earlier infected animals might play a role in the initiation of new spreadings of the infection.
3.
The nationwide control and eradication programme
In 1993, on basis of (1) the costs of the infection in cattle herds, (2) the favorable preliminary results from the pilot eradication study on the island of Samsø, (3) the results from the test evaluation study documenting that the two ELISAs were reliable and well suited for surveillance and control, the cattle farmers’ associations decided to initiate a nationwide BVD control and eradication programme as from January 1994.
The responsibility was placed with the Bovine Infectious Disease Control Board that also had been responsible for the IBR and, the final stages of, the EBL eradication programmes. There were no representatives from state institutions in this board. The Danish Cattle Health Laboratory was owned by the Danish Dairy Board, the Cattle Herd Register was still administered by the farmers’ organisations, and the Danish VeterinaryVirus Research Institute agreed to deliver special reagents necessary for the performance of the BVD antigen and antibody ELISAs.
3.1.
The situation and initiatives in early 1994
In
the first quarter of 1994, bulk tank milk samples from all 16,113
dairy herds were tested for a BVD reaction by the antibody blocking
ELISA. In Table
1
is
given an overview of the milk reactions measured and the estimated
prevalence of persistent infection in these herds. Approx. 40% of the
herds were found likely to have persistent infection. The situation
was most severe in Jutland, having both the major part of the herds
(84% of the dairy herds), and the highest average herd size.
Table 1. The initial bulk tank milk antibody ELISA reactions of the Danish dairy herds and the
corresponding probabilities of persistent infection (PI) in the first quarter of 1994.
In
the first half of 1994, the dairy herd owners were focussed on. Bulk
tank milk samples were to be tested every quarter of the year, and
examinations of milk and blood samples at the Cattle Health
Laboratory were free of charge for the farmers. A leaflet informing
about the BVD infection, the interpretation of the milk reactions,
follow-up examinations in order to further clarify the infection
status, and how to eradicate and avoid reintroduction was sent to the
farmers together with the information about the milk reaction
measured. Before that, this leaflet and another separate edition had
been sent to the veterinary practitioners, General information was
also given in farmers’ magazines.
3.2.
The full programme as from the autumn of 1994
In 1994, the Danish Veterinary Service did not yet accept to support the programme with legislative measures, so its course would be determined alone by the recommendations by the programme board and the herd owners’ understanding.
Three BVD herd infection status categories were defined, i.e., (1) most probably free, (2) undetermined, and (3) persistent infection (PI) status.
Most probably free status would be given to (1) dairy herds with two latest milk reactions below 50 percent blocking, (2) dairy and non-dairy herds, where a recommended “nine-animals” blood examination, i.e., of 3 first parity cows, 3 pregnant heifers, and 3 calves 8-10 months old, had shown no evidence of PI (the 3 calves should be both virus- and antibody-negative), but also (3) all herds, where clarifying examinations of 3 calves, usually 8-10 months old, had shown them to be virus- and antibody-negative. In herds, where a persistent infection had earlier been found, such a blood examination with a negative result would not regularly be accepted to give a free status unless performed more than one year after removal of the last persistently infected animal.
A free status should be confirmed by subsequent examinations after not more than 12 months. New leaflets on control of BVD were distributed to all dairy and non-dairy herd owners and herd advisors, but the veterinary practitioners were placed centrally. At regular intervals, the veterinary practices would receive overviews of their dairy and non-dairy herds informing about infection status, relevant results from earlier examinations, the latest date for a new blood-sampling for confirmation of the free status in non-dairy herds and dairy herds with tank milk reactions over 50 percent blocking, etc. The herd owners would also be informed directly concerning the deadline for control of free status.
In 1994, the Danish Veterinary Service did not yet accept to support the programme with legislative measures, so its course would be determined alone by the recommendations by the programme board and the herd owners’ understanding.
Three BVD herd infection status categories were defined, i.e., (1) most probably free, (2) undetermined, and (3) persistent infection (PI) status.
Most probably free status would be given to (1) dairy herds with two latest milk reactions below 50 percent blocking, (2) dairy and non-dairy herds, where a recommended “nine-animals” blood examination, i.e., of 3 first parity cows, 3 pregnant heifers, and 3 calves 8-10 months old, had shown no evidence of PI (the 3 calves should be both virus- and antibody-negative), but also (3) all herds, where clarifying examinations of 3 calves, usually 8-10 months old, had shown them to be virus- and antibody-negative. In herds, where a persistent infection had earlier been found, such a blood examination with a negative result would not regularly be accepted to give a free status unless performed more than one year after removal of the last persistently infected animal.
A free status should be confirmed by subsequent examinations after not more than 12 months. New leaflets on control of BVD were distributed to all dairy and non-dairy herd owners and herd advisors, but the veterinary practitioners were placed centrally. At regular intervals, the veterinary practices would receive overviews of their dairy and non-dairy herds informing about infection status, relevant results from earlier examinations, the latest date for a new blood-sampling for confirmation of the free status in non-dairy herds and dairy herds with tank milk reactions over 50 percent blocking, etc. The herd owners would also be informed directly concerning the deadline for control of free status.
The most important guidelines to
be observed by herd owners were that (1) only animals found
non-persistently infected after blood testing should be admitted to
the herd, but pregnant antibody-positive animals should further be
isolated at calving and the calf should be controlled before it was
admitted into the herd, (2) exhibitions, auction sales, and common
pastures should be free of persistently infected animals, and (3) on
infected farms small ruminants should also be checked for BVD, cf.
Bitsch
and Rønsholt (1995) [10].
The
course of the programme in dairy herds and non-dairy herds over the
five years from 1994 through 1998 has been published earlier [11] but
is shown
here for
the whole period up to 2002 in Figures
3
and
4.
Figure
3. The course of the BVD eradication programme for the Danish dairy
herds from 1994 to 2002.
When the first edition of this figure was created, the number of herds had been reduced from 16,113 to approx. 14,000, and by January 2002 only approx. 9,000 were active as dairy herds.
When the first edition of this figure was created, the number of herds had been reduced from 16,113 to approx. 14,000, and by January 2002 only approx. 9,000 were active as dairy herds.
Figure 4. The course of the BVD eradication programme for the Danish non-dairy cattle herds from 1994 to 2002.
The increase in the number of herds with undetermined status in April 1997 and 1999 were due to the fact that control of free status in a number of herds was conducted after the expiration of the 12 months free-status period. The effect of the first ministerial BVD order in the first quarter of 1996 is pronounced. The category of herds with undetermined status includes herds with specialized production of feeder calves for slaughter having dispensation from certain legal regulations.
3.3. Legislative measures as from 1996
In
early 1996, the State Veterinary Service agreed to issue a
ministerial order for support of the BVD programme. Persistent BVD
infection was made notifiable, and visitors and neighbours had to be
informed about the disease situation. All cattle herds should clarify
their BVD infection status, either for dairy herds on basis of
repeatedly low tank milk reactions or, generally for all other herds,
on basis of a blood test of at least three calves as practiced
earlier, but more samples, if this was not sufficient to clarify the
status as either free or persistently infected. PI animals were not
allowed at pasture, but of greatest importance was the requirement
that any animal to be moved to another herd, a common pasture, or an
exhibition had to be followed by a certificate printed out from the
herd register documenting that it had been tested on a blood sample
and found not to be persistently infected. Pregnant animals from
non-free herds were not allowed for trade. Dispensation from the
certificate requirement was given only for calves moved to herds
specialized in the production of feeder calves for slaughter.
Furthermore, a BVD free status had to be confirmed at regular intervals, either by tank milk reactions below 50 percent blocking or by an annual blood test of usually 3 animals 8-10 months old. But eradication was not made compulsory in individual herds, mainly because the State Veterinary Service was of the opinion that farmers then would require compensation from the state for expenses connected with eradication. In the final stages of the eradication programme, it became mandatory for farmers with infected herds to elaborate an eradication plan to be acknowledged by the programme administration, but still without an obligation to eradicate.
Furthermore, a BVD free status had to be confirmed at regular intervals, either by tank milk reactions below 50 percent blocking or by an annual blood test of usually 3 animals 8-10 months old. But eradication was not made compulsory in individual herds, mainly because the State Veterinary Service was of the opinion that farmers then would require compensation from the state for expenses connected with eradication. In the final stages of the eradication programme, it became mandatory for farmers with infected herds to elaborate an eradication plan to be acknowledged by the programme administration, but still without an obligation to eradicate.
3.4.
Infection of earlier free herds
New
BVD infections in earlier free herds over the first five years of the
eradication programme have been dealt with in a publication by Bitsch
et al.
(2000)
[11]. By January 1994, there were 16,113 dairy and approx. 22,000
non-dairy herds. The numbers of earlier free herds found infected in
these two herd categories from 1994 through 2001 were as follows:
1994, 1 and 0; 1995, 153 and 8; 1996, 418 and 43; 1997, 393 and 196;
1998, 204 and 129; 1999, 164 and 82; 2000, 69 and 50; and 2001, 29
and 22, respectively. In the first few years, some herds were likely
to have been falsely recorded as BVD-free, but all herds from the
later years should be judged recently infected.
The causes of infection were investigated in 67 dairy herds found infected in the one-year period from July 1997. In 17 cases (25%), no immediate cause could be found, but 19 (28%) were due to purchase of a pregnant animal delivering a persistently infected calf, 5 (7%) were caused by infection of a pregnant heifer in one and the same common pasture, and 26 (39%) were associated with the presence of persistently infected animals on a neighbouring farm or field. The many cases caused by the birth of a persistently infected calf from purchased pregnant cows or heifers gave rise to a new regulation in the 1999 BVD order that female animals over one year of age from non-BVD-free herds were not allowed to be moved from the herd except for slaughter.
The likelihood of airborne introduction from infected farms in the neighbourhood and the problem of so many cases, where the origin of infection could not readily be found, will be dealt with in more detail in Section 4.
The causes of infection were investigated in 67 dairy herds found infected in the one-year period from July 1997. In 17 cases (25%), no immediate cause could be found, but 19 (28%) were due to purchase of a pregnant animal delivering a persistently infected calf, 5 (7%) were caused by infection of a pregnant heifer in one and the same common pasture, and 26 (39%) were associated with the presence of persistently infected animals on a neighbouring farm or field. The many cases caused by the birth of a persistently infected calf from purchased pregnant cows or heifers gave rise to a new regulation in the 1999 BVD order that female animals over one year of age from non-BVD-free herds were not allowed to be moved from the herd except for slaughter.
The likelihood of airborne introduction from infected farms in the neighbourhood and the problem of so many cases, where the origin of infection could not readily be found, will be dealt with in more detail in Section 4.
.
3.5.
Costs of the BVD infection without intervention against costs of the
control and eradication programme
Houe
et al.
(1993) [12] concluded that the annual losses in Denmark due to BVD
would be approx. 101 million kroner (M Dkk).
The actual programme expenses over the first 4 years were later estimated as follows:
The actual programme expenses over the first 4 years were later estimated as follows:
- clarification of herd infection
status: 20 M Dkk
- bulk tank milk surveillance testings: 5 M Dkk
- bulk tank milk surveillance testings: 5 M Dkk
-
blood control of BVD free status: 32
M Dkk
- eradication in herds: 53 M Dkk
- certificate testings: 90 M Dkk
Total, for the first four years: 200 M Dkk
- eradication in herds: 53 M Dkk
- certificate testings: 90 M Dkk
Total, for the first four years: 200 M Dkk
And
the annual programme expenses for the first few years thereafter were
estimated to be as follows:
-
bulk tank milk surveillance testings:
1
M Dkk
-
blood control of BVD free status:
16
M Dkk
- continued eradication in herds: 5 M Dkk
- certificate testings: 8 M Dkk
- continued eradication in herds: 5 M Dkk
- certificate testings: 8 M Dkk
Total,
per year: 30 M Dkk
From
these three estimates, it will follow that the expenses connected
with the control and eradication of BVD with a gradual decline in
losses from the infection, cf. Figures
3 and 4,
would have been paid back after less than 8 years.
Bulk tank milk samples were tested 4 times a year, and the extent of the blood examinations over the years from 1994 through 2000 is shown in Figure 5.
Bulk tank milk samples were tested 4 times a year, and the extent of the blood examinations over the years from 1994 through 2000 is shown in Figure 5.
Figure 5. Blood samples tested in the Danish BVD control and eradication programme 1994-2000.
All samples were tested for antibodies and most of them also for the virus. The increased numbers tested January-June 1996 were a response to the first ministerial BVD order.
4.
Discussion and conclusions
General considerations
As
mentioned above, BVD was widespread in Denmark prior to 1992 to such
an extent that hardly any dairy herd was without antibody carriers.
Animal trade was common and closed herds were extremely rare, Not
even the successful IBR eradication initiated 1984 had changed the
general habits of the farmers, most probably because the IBR
programme lines and the test system were so reliable that uninfected
animals had been allowed for sale from even infected herds.
Eradication of BVD would be costly, and it was of extreme importance
that test methods were safe and suited for automation and that the
lines selected for control and eradication were effective. No other
countries had experiences from the control of BVD, and none with a
similarly high prevalence of the infection seems even to have
considered this option. No unforeseen problems should arise after the
start of eradication. A second chance, if the first attempt should
fail, would be unlikely. Consequently, it was found that pilot
studies were necessary and had to be comprehensive and targeted to
clarify all major questions.
It was a huge advantage that the farmers had their own laboratory with any necessary professional expertise, and that the central herd register could easily be used to handle all BVD data including laboratory registrations, reports, and certificates. The laboratory capacity was never a problem and could easily have been further enlarged.
The main conclusions from the pilot studies have been given in Section 2. But it is worth recapitulating that the tests used were optimal. The antigen ELISA never failed, an antibody reaction in a serum or plasma sample of more than 50% of blocking was always truly positive, and it is impossible to recollect an instance, where an antibody reaction in tank milk of more than 20% of blocking did not appear to be specific. It is important to realize that the basic requirement to a surveillance test on bulk tank milk is that a reaction is recorded as soon as a new infection has been spread among the cows. If that requirement is met with, the weakness of a bulk milk test will only be the length of the interval between testings. For BVD, tests performed 4 times a year were satisfactory, but for IBR we had to change to monthly testings in the winter seasons in the south of Jutland in the last years of the eradication phase and the first years after eradication, because of the airborne introduction of infection from our neighbour country.
After 2000, several countries outside the Nordic countries have shown interest in the eradication of BVD, but apparently often with unsatisfactory results. In 2008, however, Switzerland changed its programme completely and started to test all cattle for persistent infection over a relatively short period. This scheme minimizing the risk of new infections during the eradication period has been effective (Schwermer et al., 2016) [13]. Other countries like Germany (from 2011) (Wernike et al., 2o17) [14] and Ireland (from 2013) (Thulke et al., 2018) [15] have followed the line to test newborn calves in connection with ear-tagging, but seem to have been less restrictive regarding the testing of older animals in infected herds, and in Germany vaccination is still allowed in order to reduce the risk of reinfection.
Many more countries will consider BVD eradication, and the experiences from the successful eradication in Denmark, densely populated with cattle and with an initial very high prevalence of the infection, will be important for the decision-makers anywhere.
It was a huge advantage that the farmers had their own laboratory with any necessary professional expertise, and that the central herd register could easily be used to handle all BVD data including laboratory registrations, reports, and certificates. The laboratory capacity was never a problem and could easily have been further enlarged.
The main conclusions from the pilot studies have been given in Section 2. But it is worth recapitulating that the tests used were optimal. The antigen ELISA never failed, an antibody reaction in a serum or plasma sample of more than 50% of blocking was always truly positive, and it is impossible to recollect an instance, where an antibody reaction in tank milk of more than 20% of blocking did not appear to be specific. It is important to realize that the basic requirement to a surveillance test on bulk tank milk is that a reaction is recorded as soon as a new infection has been spread among the cows. If that requirement is met with, the weakness of a bulk milk test will only be the length of the interval between testings. For BVD, tests performed 4 times a year were satisfactory, but for IBR we had to change to monthly testings in the winter seasons in the south of Jutland in the last years of the eradication phase and the first years after eradication, because of the airborne introduction of infection from our neighbour country.
After 2000, several countries outside the Nordic countries have shown interest in the eradication of BVD, but apparently often with unsatisfactory results. In 2008, however, Switzerland changed its programme completely and started to test all cattle for persistent infection over a relatively short period. This scheme minimizing the risk of new infections during the eradication period has been effective (Schwermer et al., 2016) [13]. Other countries like Germany (from 2011) (Wernike et al., 2o17) [14] and Ireland (from 2013) (Thulke et al., 2018) [15] have followed the line to test newborn calves in connection with ear-tagging, but seem to have been less restrictive regarding the testing of older animals in infected herds, and in Germany vaccination is still allowed in order to reduce the risk of reinfection.
Many more countries will consider BVD eradication, and the experiences from the successful eradication in Denmark, densely populated with cattle and with an initial very high prevalence of the infection, will be important for the decision-makers anywhere.
Legislation
Figure
4
documents
that the non-dairy cattle farmers were reluctant to join the
programme during the first two years. Due to the presence of the
infection in these herds, eradication in dairy herds was therefore
severely hampered, cf. Bitsch
et al.
[11]. Only legal provisions could solve this problem, and after two
years the veterinary authorities agreed to support the programme with
legislation. They did not want, however, to make eradication
obligatory but accepted to issue provisions aiming at preventing the
transmission of the infection between herds. The most important
regulations were (1) that animals to be moved to other herds, to
exhibitions or common pastures had to be followed by a certificate
issued on basis of blood testing of the individual animals, (2) that
no persistently infected animals were allowed on pastures, and (3)
that all herds should clarify their infection status.
A new BVD order was issued almost every year. The 1999 edition contained both a tightening and relaxation. Because too many farmers did not follow the recommendation to isolate pregnant animals bought from other herds at calving and to test the calf before it was admitted to the herd, no female animals over one year of age from non-BVD-free herds were now allowed to be sold to other herds. But most importantly, trade certificates for animals from herds having been BVD-free for more than two years could now be issued without blood testing of the individual animals. Also, minor changes were introduced, for example, that a representative number of antibody-negative animals after the return from common pastures should be controlled for antibodies.
After the first five years, because of the many new cases of infected herds, it was concluded that a solution should be found to make eradication mandatory, cf. Bitsch et al. (2000) [11], but the Danish Veterinary Service maintained up to 2002 that this was not an option.
A new BVD order was issued almost every year. The 1999 edition contained both a tightening and relaxation. Because too many farmers did not follow the recommendation to isolate pregnant animals bought from other herds at calving and to test the calf before it was admitted to the herd, no female animals over one year of age from non-BVD-free herds were now allowed to be sold to other herds. But most importantly, trade certificates for animals from herds having been BVD-free for more than two years could now be issued without blood testing of the individual animals. Also, minor changes were introduced, for example, that a representative number of antibody-negative animals after the return from common pastures should be controlled for antibodies.
After the first five years, because of the many new cases of infected herds, it was concluded that a solution should be found to make eradication mandatory, cf. Bitsch et al. (2000) [11], but the Danish Veterinary Service maintained up to 2002 that this was not an option.
On
small ruminants as a source of infection for cattle
To
rule out that free-living deer could be a source of infection for
cattle in pastures, Nielsen
et al.
[16] investigated 476 blood samples collected 1995-1999 from deer in
various parts of the country. They were tested for virus and antibody
by the blocking ELISA at the Danish Cattle Health Laboratory, and
only 3 were found antibody-positive. These samples were also positive
in a standard virus neutralization test. It was concluded that the
infected animals had received the infection from cattle, and that
neither the BVD infection nor another pestivirus infection was likely
to be maintained in the Danish deer population.
The question, if small domestic ruminants might be a source of infection for cattle was more complex. Bitsch et al. [11] reported that a ram on an infected cattle farm had been found persistently infected and most probably had even introduced the infection on the farm a couple of years earlier. The general guidelines for eradication on infected cattle farms, however, included control of sheep and goats being present, so infected sheep should not be a problem in connection with eradication in herds, but still they might cause new infections in cattle herds. Tegtmeier et al. 2000 [17] examined 2 blood samples from each of 1000 sheep and goat herds for antibody to what was called border disease, and found antibody in 6.3% of the approx. 850 sheep herds. They found a positive correlation between antibody response and presence of cattle on the farm but did not include infection status of the cattle herds, and the number of samples from each herd was too low to rule out the possibility of a separate and independent BVD or border disease infection in sheep. Hansen and Bitsch [18] tested, mainly in the winter of 1999-2000, an average of 10 samples from 122 herds divided into two groups, namely (1) flocks on 39 premises where persistent BVD infection had been diagnosed in cattle in recent years, and (2) flocks on 83 premises without cattle. In all, 16 herds (49%) in the group with earlier persistently infected cattle on the farm showed reaction in at least one sample. Of the 307 animals tested in these flocks, 45 (15%) were found antibody-positive. In contrast hereto, only 2 flocks (2,4%) in the group of 83 premises without cattle were found with antibody carriers. Of 818 animals, 5 were positive (0. 6%). The two flocks had 2 and 3 antibody carriers out of ten animals tested, their age was 3-4 years, and the flock sizes were 92 and 24 animals. The antibody reactions were confirmed in a conventional virus neutralization test. It was concluded (1) that although sheep could easily be infected by the BVD virus, BVD was not regularly a self-maintained infection in sheep, and (2) that there were no indications, whatsoever, of border disease being present in sheep in Denmark.
The question, if small domestic ruminants might be a source of infection for cattle was more complex. Bitsch et al. [11] reported that a ram on an infected cattle farm had been found persistently infected and most probably had even introduced the infection on the farm a couple of years earlier. The general guidelines for eradication on infected cattle farms, however, included control of sheep and goats being present, so infected sheep should not be a problem in connection with eradication in herds, but still they might cause new infections in cattle herds. Tegtmeier et al. 2000 [17] examined 2 blood samples from each of 1000 sheep and goat herds for antibody to what was called border disease, and found antibody in 6.3% of the approx. 850 sheep herds. They found a positive correlation between antibody response and presence of cattle on the farm but did not include infection status of the cattle herds, and the number of samples from each herd was too low to rule out the possibility of a separate and independent BVD or border disease infection in sheep. Hansen and Bitsch [18] tested, mainly in the winter of 1999-2000, an average of 10 samples from 122 herds divided into two groups, namely (1) flocks on 39 premises where persistent BVD infection had been diagnosed in cattle in recent years, and (2) flocks on 83 premises without cattle. In all, 16 herds (49%) in the group with earlier persistently infected cattle on the farm showed reaction in at least one sample. Of the 307 animals tested in these flocks, 45 (15%) were found antibody-positive. In contrast hereto, only 2 flocks (2,4%) in the group of 83 premises without cattle were found with antibody carriers. Of 818 animals, 5 were positive (0. 6%). The two flocks had 2 and 3 antibody carriers out of ten animals tested, their age was 3-4 years, and the flock sizes were 92 and 24 animals. The antibody reactions were confirmed in a conventional virus neutralization test. It was concluded (1) that although sheep could easily be infected by the BVD virus, BVD was not regularly a self-maintained infection in sheep, and (2) that there were no indications, whatsoever, of border disease being present in sheep in Denmark.
Infection
of earlier free herds
In
Section
3.4
are
given the eventually declining numbers of new infected, but earlier
free, dairy- and non-dairy herds over the years together with data
from an analysis of causes of infection in 67 herds found infected
after June 1997. Although the expenses connected with the eradication
of the infection in such herds generally are reasonably low, the
numbers of new infected herds were too high to be neglected.
The ban on purchase of female animals over one year of age from herds with a non-BVD-free status only solved a minor part of the problem.
In a relatively high number of the 67 herds investigated thoroughly, no cause of infection could readily be found. Iatrogenic infection, as documented for Aujeszky’s disease and IBR, was not likely to be accountable for more than a very few of them. Reactivation of the infection in a pregnant animal and subsequent transmission to its fetus was thought likely [10], but only one such possible case was observed during the eradication period. Here a heifer, having been found antibody-positive at the age of more than one year just as the other animals of that age, delivered a persistently infected calf, in spite of the fact that she was inseminated after that the herd had been cleared of the infection. Such a possibility must, therefore, be extremely rare and of no practical importance.
The bulls at the AI centres were free of persistent infection, but Voges et al. (2000) [19] reported having demonstrated persistent BVD virus infection in the testes of an antibody-positive bull leading to the presence of virus in the semen. Bitsch et al. (2000) [11] investigated, therefore, register data for 533 infected but earlier BVD-free dairy herds. In 251 herds, 291 calves were identified as the ones primarily infected, while 2633 others persistently infected were identified as secondarily infected, but no bulls were more frequently represented as fathers of the primary persistently infected calves than others, so the presence of a bull shedding virus in its semen at AI centres was found highly unlikely.
Many new cases were associated with the presence of persistently infected animals on a neighboring farm, and some of them had even been admitted to pastures. Airborne transmission over distances over up to several kilometers had been a severe problem in connection with the eradication of both Aujeszky’s disease and IBR (dealt with in detail in a review by Bitsch [4]). In this context, it should be realized that viral infections of the respiratory tract are generally spread in two ways, i.e., (1) among individuals in close contact and (2) airborne over many meters by air currents, which in animal houses usually are directed by the ventilation systems. This airborne spread is of utmost importance, first because it is responsible for the rapid spread of the infection within a herd, and second because the amounts of virus sent out into the open air by the ventilation system will expose other herds to a risk of infection. Bitsch and Rønsholt [10] argued in their paper from 1995 that even if there would usually be only a single or relatively few persistently infected animals in a herd to shed considerable amounts of virus, the risk of transmission to other herds would, nevertheless, be relatively high, because they would shed virus for their whole lifetime.
The ban on purchase of female animals over one year of age from herds with a non-BVD-free status only solved a minor part of the problem.
In a relatively high number of the 67 herds investigated thoroughly, no cause of infection could readily be found. Iatrogenic infection, as documented for Aujeszky’s disease and IBR, was not likely to be accountable for more than a very few of them. Reactivation of the infection in a pregnant animal and subsequent transmission to its fetus was thought likely [10], but only one such possible case was observed during the eradication period. Here a heifer, having been found antibody-positive at the age of more than one year just as the other animals of that age, delivered a persistently infected calf, in spite of the fact that she was inseminated after that the herd had been cleared of the infection. Such a possibility must, therefore, be extremely rare and of no practical importance.
The bulls at the AI centres were free of persistent infection, but Voges et al. (2000) [19] reported having demonstrated persistent BVD virus infection in the testes of an antibody-positive bull leading to the presence of virus in the semen. Bitsch et al. (2000) [11] investigated, therefore, register data for 533 infected but earlier BVD-free dairy herds. In 251 herds, 291 calves were identified as the ones primarily infected, while 2633 others persistently infected were identified as secondarily infected, but no bulls were more frequently represented as fathers of the primary persistently infected calves than others, so the presence of a bull shedding virus in its semen at AI centres was found highly unlikely.
Many new cases were associated with the presence of persistently infected animals on a neighboring farm, and some of them had even been admitted to pastures. Airborne transmission over distances over up to several kilometers had been a severe problem in connection with the eradication of both Aujeszky’s disease and IBR (dealt with in detail in a review by Bitsch [4]). In this context, it should be realized that viral infections of the respiratory tract are generally spread in two ways, i.e., (1) among individuals in close contact and (2) airborne over many meters by air currents, which in animal houses usually are directed by the ventilation systems. This airborne spread is of utmost importance, first because it is responsible for the rapid spread of the infection within a herd, and second because the amounts of virus sent out into the open air by the ventilation system will expose other herds to a risk of infection. Bitsch and Rønsholt [10] argued in their paper from 1995 that even if there would usually be only a single or relatively few persistently infected animals in a herd to shed considerable amounts of virus, the risk of transmission to other herds would, nevertheless, be relatively high, because they would shed virus for their whole lifetime.
It will always be
difficult to document that an infection has been transmitted by the
airborne mode. The evidence will practically always be
circumstantial. But both in the case of Aujeszky's disease and IBR,
the overwhelming evidence of airborne herd-to-herd transmissions was
confirmed by the fact that, after the final eradication, new
infections were regularly introduced in the winter periods in border
areas close to Germany, where the only possible source of infection
was infected German herds. In many of these instances, the infection
was even spread further to neighbouring Danish herds, cf. review [4].
With respect to the group of new infected herds where no immediate cause of infection could be found, the infection had most probably been introduced by the airborne mode in the instances where persistently infected animals were present on neighbouring farms. But as no other imaginable cause of infection could be found for the rest of herds in this group, it will be justified to conclude that airborne transmission over longer distances most likely was responsible also for most of these cases.
It is evident from the details above that the many cases of infection of free herds over the years were caused by introduction from herds with an active BVD infection. Consequently, eradication should have been compulsory right from the start of the programme. If all herds had eradicated the infection over the first two or three years, for example regionwise, numerous new cases would have been avoided and many expenses saved. And the eradication phase would have become much shorter, most probably below 4 years.
With respect to the group of new infected herds where no immediate cause of infection could be found, the infection had most probably been introduced by the airborne mode in the instances where persistently infected animals were present on neighbouring farms. But as no other imaginable cause of infection could be found for the rest of herds in this group, it will be justified to conclude that airborne transmission over longer distances most likely was responsible also for most of these cases.
It is evident from the details above that the many cases of infection of free herds over the years were caused by introduction from herds with an active BVD infection. Consequently, eradication should have been compulsory right from the start of the programme. If all herds had eradicated the infection over the first two or three years, for example regionwise, numerous new cases would have been avoided and many expenses saved. And the eradication phase would have become much shorter, most probably below 4 years.
Costs
and benefits of the eradication programme
The
programme was elaborated mainly on the basis of the results from the
pilot studies and the experiences from the IBR eradication scheme
aiming at achieving high safety at low costs. It is evident that the
fact that the scheme was without legislative support during the first
two years both delayed the eradication and made it more costly
because of reduced efficacy.
That a cost-benefit estimation, nevertheless, was positive after the elapse of less than 8 years underlines that BVD should be controlled everywhere. In this context, it will be relevant to mention that really many farmers reported back after the eradication that the health condition among the calves had become better than ever before. So it seems that the presence of persistent BVD infection generally gives rise to secondary disease problems not usually attributed to the BVD virus. Last, but not least, it should be realized that animal welfare alone is a high priority reason for control of a disease like BVD.
That a cost-benefit estimation, nevertheless, was positive after the elapse of less than 8 years underlines that BVD should be controlled everywhere. In this context, it will be relevant to mention that really many farmers reported back after the eradication that the health condition among the calves had become better than ever before. So it seems that the presence of persistent BVD infection generally gives rise to secondary disease problems not usually attributed to the BVD virus. Last, but not least, it should be realized that animal welfare alone is a high priority reason for control of a disease like BVD.
References
-
The Danish Veterinary Service. The national animal health and disease control position in
Denmark 1991. ISSN 0109-7849. -
Verdugo C, Toft N, Nielsen SS. Within- and between-herd prevalence variation of Mycobacterium avium subsp. paratuberculosis infection among control programme herds in Denmark (2011–2013). Prev. Vet. Med. 2015; 121: 282-287.
-
Nielsen LR. Salmonella Dublin in cattle. 2013. Thesis. The University of Copenhagen. ISBN 978-87-7611-611-8.
-
Bitsch V. Principal epidemiological features of Aujeszky's disease - Suid herpesvirus 1 infection - in swine and cattle. ISBN 978-87-994685-1-5. Available from: http://suhv1epidemiology.blogspot.com/
-
Rønsholt L, Nylin B, Bitsch V. A BVD antigen and antibody-blocking ELISA system used in the Danish voluntary eradication programme. Proceedings of the 3rd Symposium on Pestiviruses, Sept. 1996, Lelystad, Netherlands, ESVV. 1997; 150-153.
- Kamstrup S, Rønsholt L,Jensen, MH, Dalsgård K. A novel subunit ISCOM vaccine against bovine virus diarrhea virus. Rev. Sci. Tech. 1992; 3: 873-877.
-
Bitsch V. The regular lines of antigen-antibody interactions in vitro. ISBN 978-87-994685-2-2. Available from: http://antigenantibodyinteractions.blogspot.com/
-
Bitsch V, Houe H, Rønsholt L, Madsen KF, Valbak J, Rough NH, Eckhardt CH. På vej mod kontrol af BVD (Control of BVD is underway. With a summary in English.) Dansk Vet-Tidsskr. 1994; 77: 445-450.
-
Bitsch V, Houe H, Nylin B, Rønsholt L. Examination of blood and bulk tank milk samples to monitor the bovine virus diarrhea infection status of cattle herds. Proceedings of the 3rd Symposium on Pestiviruses, Sept. 1996, Lelystad, Netherlands, ESVV. 1997; 158-161.
-
Bitsch V, Rønsholt L. Control of bovine viral diarrhea infection without vaccines. The Vet. Clin. North Am., Food Anim. Pract. 1995; 11: 521-547.
-
Bitsch V, Hansen K-EL, Rønsholt L. Experiences from the Danish programme for eradication of bovine virus diarrhea (BVD) 1994-1998 with special reference to legislation and causes of infection. Vet. Microbiol. 2000; 77: 137-143.
-
Houe H, Pedersen KM, Meyling A. A computerized spreadsheet model for calculating total annual national losses due to bovine viral diarrhea virus infection in dairy herds and sensitivity analysis of selected parameters. Proceedings of the Second Symposium on Pestiviruses, Annecy, France. 1993; 179-184.
-
Schwermer H, Di Labio E. From control to surveillance – the Swiss bovine viral diarrhoea (BVD) eradication programme. 2016. Proc. 29th World Buiatric Congress, Dublin.
-
Wernike K, Gethmann J, Schirrmeier H, Schröder R, Conraths FJ, Beer M. Six years (2011-2016) of mandatory nationwide bovine viral diarrhea control in Germany – a success story. Pathogens. 2017; 6: 50.
-
Thulke H-H, Lange M, Tratalos JA, Clegg TA, McGrath G, O'Grady L et al. Eradicating BVD, reviewing Irish programme data and model predictions to support prospective decision making. Prev.Vet. Med. 2018; 150: 151-161.
-
Nielsen SS, Rønsholt L, Bitsch V. Bovine virus diarrhea virus in free-living deer from Denmark. J. Wildl. Dis. 2000; 36: 584-587.
-
Tegtmeier C, Stryhn H, Uttenthal A, Kjeldsen, AM, Nielsen TK. Seroprevalence of border disease in Danish sheep and goat herds. Acta vet. Scand. 2000; 41: 339-344.
-
Hansen K-EL, Bitsch V. Seroreaction in sheep in Denmark to pestivirus is caused by bovine virus diarrhoea virus. Report. 2000. Not published in official journals.
- Voges H, Horner GW, Rowe S. Persistent bovine pestivirus localized in the testes of an immuno-competent bull. Vet. Microbiol. 2000; 68: 165-175.